Antennas
|
|
We are still focused on our quest of understanding Why Is The Sky Blue? We understand white light is the sum of all colors of light, and the sunlight hits the atmosphere's molecules, which causes the electrons to move like electric current. We now want to understand why this radiates blue light, so we will learn a little bit about antenna theory.
AntennasThe simplest type of antenna is known as a dipole antenna. This is simply a thin wire antenna, with some method of forcing current to flow on the antenna, as shown in Figure 1:
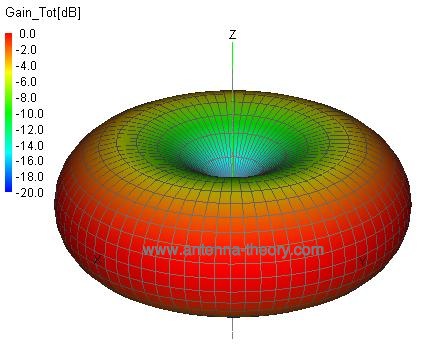
 Figure 1. A Short Dipole Antenna With a Current Source at the Center. In Figure 1, the dipole antenna is simply a wire (copper, aluminum, or any metal). To radiate energy, there must be a method of applying electric current onto the wire. This is the reason for the center fed current source shown in Figure 1. Examples of dipole antennas include TV antennas ("rabbit ears") and the AM/FM radio antenna on cars. Note that the source of energy for the molecules that become antennas is the sun's light rays that induces the electric current within the molecules. The dipole antenna of Figure 1 has a donut-shaped radiation pattern. This means the antenna will radiate in all directions, with a peak broadside to the dipole (and with minimum radiation along the length of the dipole):
Figure 2. Radiation Pattern of Dipole Antenna. We can illustrate why the atmosphere scatters light in every direction now. Imagine the trillions of molecules in the atmosphere, each floating at random directions, and each being acted upon by sunlight. Each molecule then re-radiates energy like a dipole, and we have scattering in all directions:
Figure 3. Illustration of the Scattering of Sunlight Due to Molecules in the Atmosphere. This explains where the light comes from when we look at the sky during the daytime. We are now moving forward to explain why the light turns blue when it starts out white. The most important parameter of the dipole antenna is the total length of the antenna, L. For the dipole antenna to be an efficient radiator, what size should the antenna be? It turns out that the dipole antenna should be half of a wavelength long at the frequency it would like to radiate at. If the antenna size is less than this, then the antenna radiation efficiency will be reduced. Let's talk about the antenna size again - the dipole antenna should be one half of a wavelength long. If the dipole antenna is to radiate a TV signal at 300 MHz (300 MHz has a wavelength of 1 meter), the dipole antenna should ideally be L=0.5 meters. If the dipole antenna is to radiate a WIFI signal at 2.4 GHz (2.4 GHz has a wavelength of 0.125 meters), the WIFI dipole antenna should be about L=6.25 centimeters (cm). The last paragraph illustrates that the size of the dipole antenna should be longer for lower frequencies. Higher frequency dipole antennas can be smaller. Let's now talk about molecules in the atmosphere and the wavelength of visible light. The oxygen molecules are about 100-200 picometers long (this is about 10^-10 meters - or about a billionth of a centimeter). The wavelength of red light is 650 nanometers (6.5*10^-7 meters), and that of blue light is 475 nanometers (4.5*10^-7 meters). The last paragraph is very important. It means the molecules of the atmosphere are very small relative to the wavelength of light. This means that when the molecules are excited by the light, the molecule's electrons begin to oscillate, creating an oscillating electric current. This oscillating current behaves like a short dipole antenna. The molecules are not even close to a half-wavelength long at light frequencies. This means they will radiate, but will not radiate energy very well. So what happens if the antenna is very short, and you want to radiate energy at two frequencies - say the frequency of red and blue light? The length of the molecules are about 0.01% of a wavelength for red light, and 0.022% of a wavelength for blue light. This means the molecules are better radiators at the blue light frequency than they are at the red light frequency.
How much better is the radiation at blue light than at red light? For short dipole antennas,
the radiation efficiency increases as the square of the frequency ( However, this is not the whole story. We need to figure out how much energy the molecules collect from the incident sunlight.
Radar Cross SectionHow much energy does the molecules pick up from the sunlight? This is also a function of the size of the molecule relative to the wavelength of light. This can be calculated and is known as the radar cross section - a term developed by the radar people in determining reflected energy of objects.
The molecule "looks" bigger at blue light than at red light, because the wavelength is shorter for blue
light and the molecule has a fixed physical size. If we go through all the calculations in Maxwell's
Equations, or get experimental results, we will see that the amount of energy collected by a small
molecule also increases with the square of the frequency (
This means that the total re-radiated power from a floating oxygen or nitrogen molecule will
increase as the fourth power of frequency (
Figure 4. Relative Re-Radiated Power Versus Frequency. As you can see from Figure 4, the radiated power of blue light will be significantly stronger than the radiated power from red light. There is still some red and green light in there, but the blue is stronger. Now we have learned a whole lot. We know that the sun's light is made up of all colors of the rainbow, we know that the molecules in the Earth's atmosphere collect and re-radiate this light like small dipole antennas with a relative energy as shown in Figure 4. We are almost all the way there, but Figure 4 shows us we still have one more problem: if Figure 4 is true (which it is), why is the sky not purple? This is the topic of the next section: The Sunlight Spectrum and the Human Eye.
Copyright - This explanation on the antenna theory related to why is the sky blue is
copyrighted, but can be reproduced with no permission as long as the source is referenced.
Copyright antenna-theory.com, 2013-2021.
|
1. Why Is The Sky Blue?
2. White Light 4. Antennas |

 ).
This means that blue light (f=667 THz) will be more efficient than red light (f=460 THz) by
a factor of 2.1. This can be found experimentally through
).
This means that blue light (f=667 THz) will be more efficient than red light (f=460 THz) by
a factor of 2.1. This can be found experimentally through
 ). This means a lot more blue light
will radiate than red light. If we imagine a set of molecules
re-radiate 1mW of power at the red light frequency (460 THz), we can plot the radiated power
at the other frequencies as shown in Figure 4:
). This means a lot more blue light
will radiate than red light. If we imagine a set of molecules
re-radiate 1mW of power at the red light frequency (460 THz), we can plot the radiated power
at the other frequencies as shown in Figure 4:
